By Karen Roman
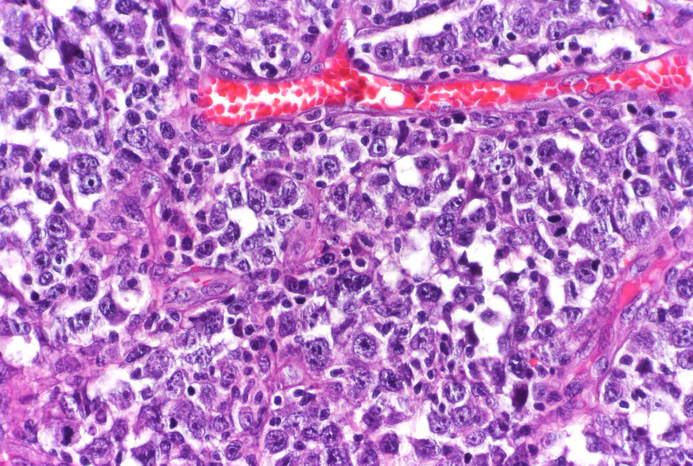
Ascentage Pharma Group Internat (Nasdaq: AAPG) said its treatment candidate for B-cell diseases APG-3288 was granted the investigational new drug clearance by the FDA.
The company will also conduct a global Phase I study evaluating APG-3288 in patients with relapsed/refractory B-cell malignancies like B-cell lymphoma and chronic lymphocytic leukemia, among others, the company stated.
“This FDA clearance marks a major milestone for the development of APG-3288 and strategic pipeline expansion that underscores our persistent innovation in the field of hematologic malignancies,” said Yifan Zhai, M.D., Ph.D., Ascentage Pharma’s Chief Medical Officer.
READ MORE
CorpGov to Host On-Site Interviews at ICR Conference 2026
Register for our weekly newsletter HERE
Contact:
Click HERE to follow us on LinkedIn






